Chemical and physical changes.
This is the first of a series of videos where I will try to cover all the necessary aspects of coffee roasting.
The objective, is to give you the foundation.
So you could build up the skills, to become a prolific coffee roaster. Regardless your equipment and budget.
Basically, by roasting it we are conditioning the coffee for human consumption.
During roasting coffee beans will experience physical and chemical changes, while they go through different stages.
Those stages are described as as follows…
Dehydration 90 C° – 194 F°
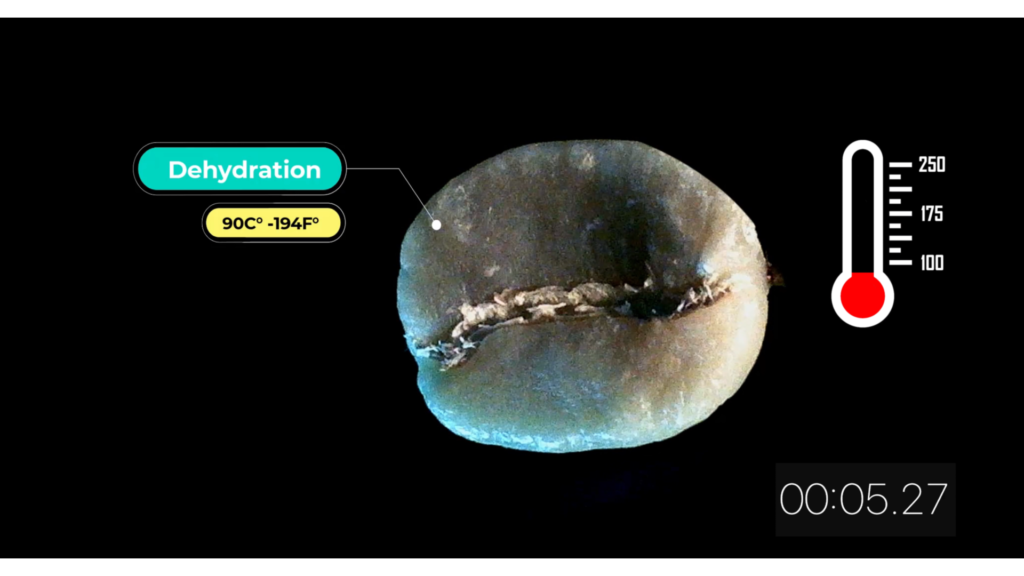
Bean color starts to change from green to yellow and moisture drops between 2 – 3%. Causing weight reduction and smell similar to toasted bread.
The silver covering starts to separate from bean’s surface and carbohydrates begin to reduce.
During the entire roasting process, we should expect between 13 – 20% in weight lost due to dehydration.
Hydrolysis 120 C° – 248 F°
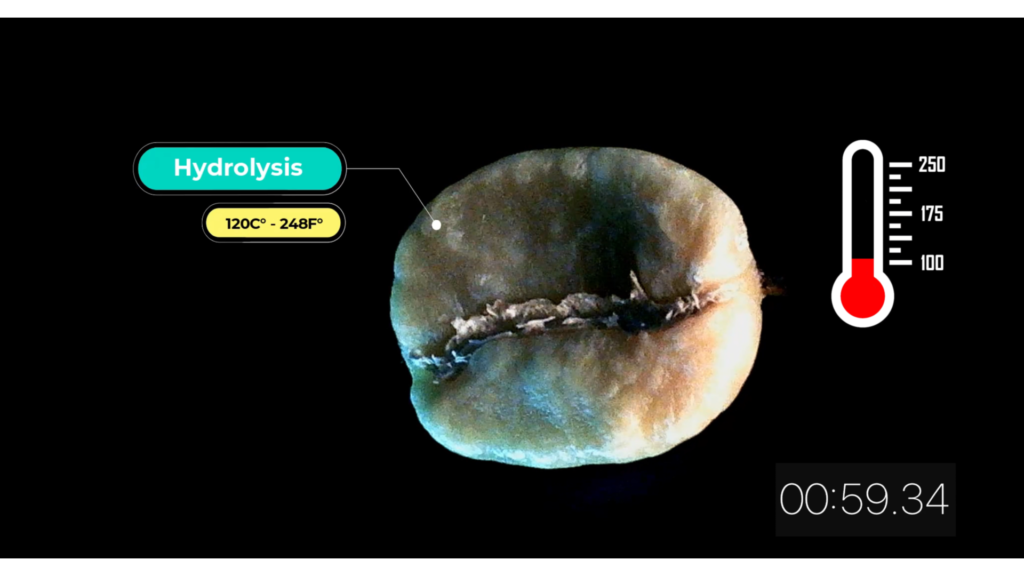
As the temperature rises water start to react with salts. Coffee beans turn to chestnut brown color.
At the same time, water ions H+ and OH- react with weak bases or acidic compounds forming hydrolyzed compounds, which in turn react with one another to form new solutions. Among them coffee acidity.
SEE ALSO: Why should you start roasting your own coffee?
Desmolysis 130 C° – 266 F°
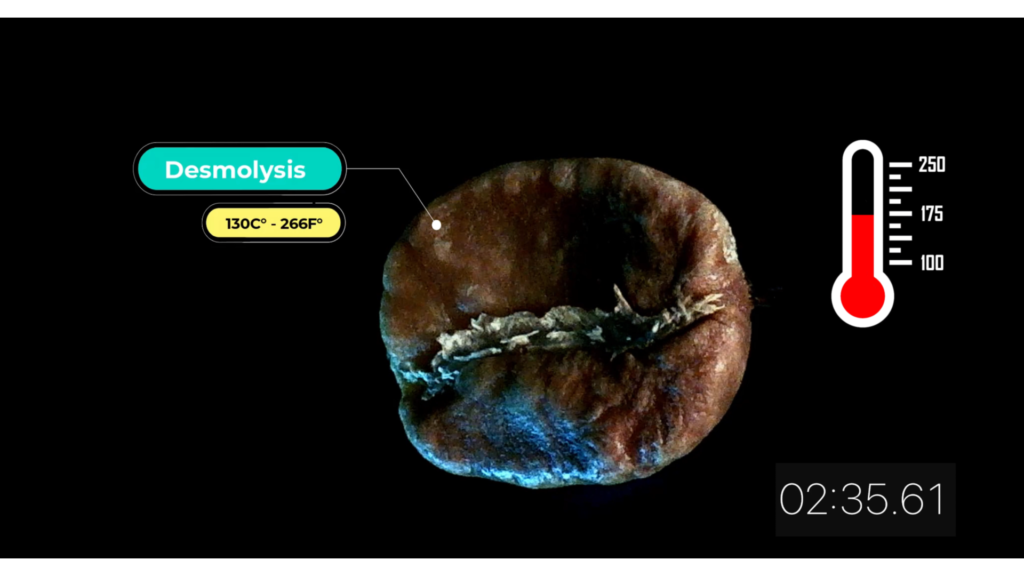
Desmolysis consists in a series of enzymatic processes resulting in the breakdown of carbon chain links. Sugars and starches begin to transform, contributing to a large portion of the taste and coffee aroma.
At 150 – 170°C (302-338°F) coffee begins to smell like roasted bean.
Catalysis 150 C° – 302 F°
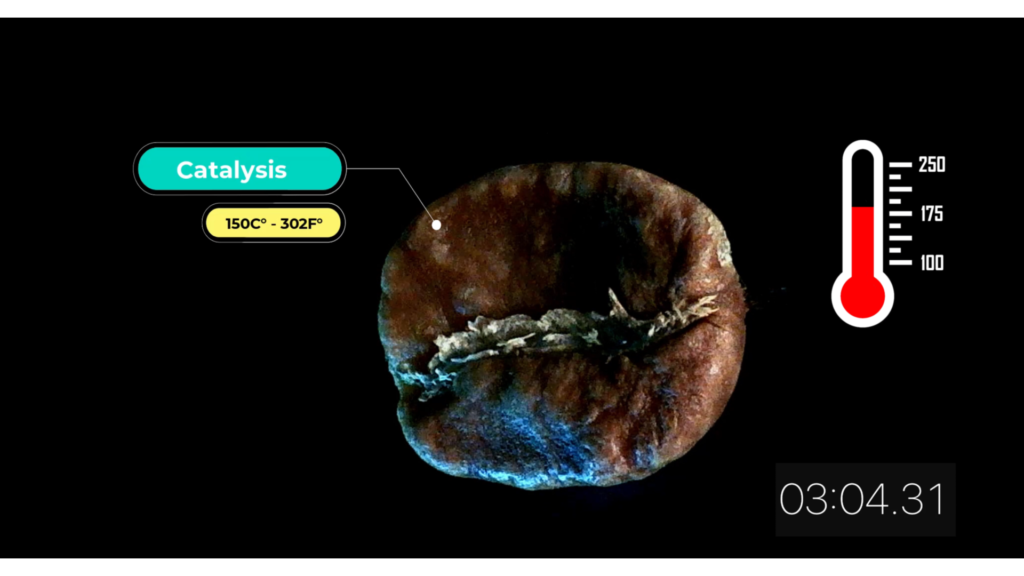
We start perceiving the distinctive aroma of roasted coffee. Combustion gasses are released (CO2 and CO). Beans’ color turns to a darker brown and volume increases between 40-60%.
Once, the temperature inside the beans gets close to 100°C (212°F) the moisture inside the bean is suddenly transformed into gas and the pressure is released producing a cracking noise.
This is known as the first crack.
Caramelization
Caramelization is the oxidation or thermal decomposition of sugars into color and flavor precursors and take place between 160-170˚C (320-338°F).
Thermal destabilization, causes sugars to produce two different substances. Compounds with low molecular weight, formed by dehydration and cyclization. Which constitute between 5-10% of the total volatile molecules and cause the typical caramel smell and taste.
Hydroximetil-furfural (HMF) and hydroxiacetil-furan (HAF) are responsible for the distinctive caramel colors as they polymerize. As the caramelization progresses sugar polymers in a varied range and complexity are produced. They constitute between 90-95% of the total. Mostly polydextrose and oligosaccharides of glucose.
However, the most typical products of caramelization are: fructose dianhydrides (DAF) or fructose and glucose mixes.
Millard reactions
Millard reactions are non-enzymatic chemical reaction between amino acids and reducing sugars. Free amino acids, derived from peptides and proteins react with reducing sugars forming nitrogen heterocycles
and polymeric melanoidins, after condensation they give roasted coffee beans their dark brown color.
Above 150°C (302°F), Maillard reactions induce free proteins in coffee to combine with reducing sugars, generating hundreds of important aromatic components. Pyrazines and pyridines contribute greatly and
they are responsible for notes of walnut, baked cereals or toasted bread found in coffee.
When internal temperature of the beans reaches 190°C (374°F).
Bean’s oils suddenly turn into gas. and the pressure is release through an audible cracking sound. (2nd crack) exposing the oils on the bean surface.
Therefore, acquiring a darker and shiny appearance .
Pyrolysis 190 C° – 374 F°
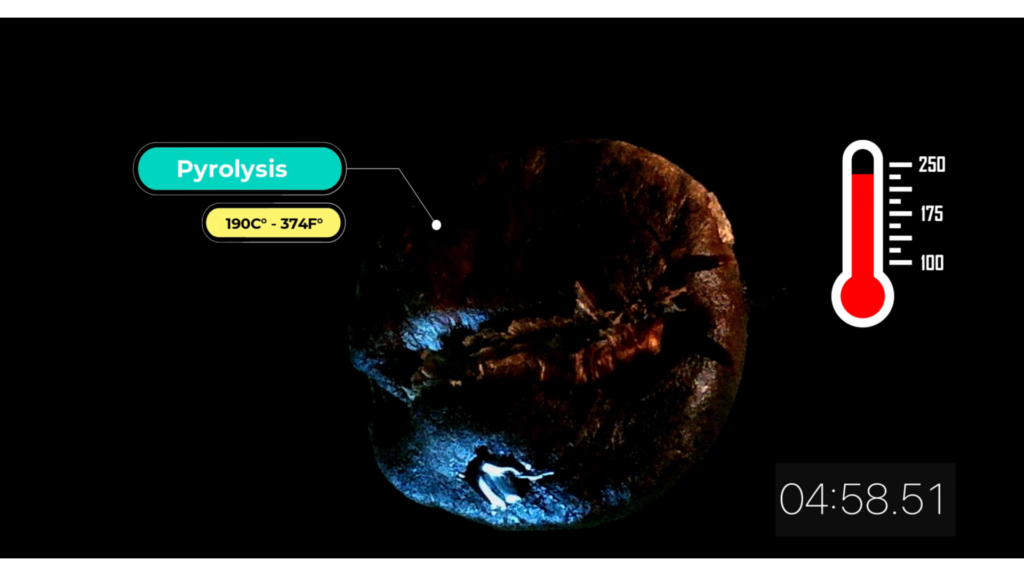
Pyrolysis or dry distillation is a physical and chemical process that transforms organic matter into high energy content products. It involves heating organic matter in the absence of oxygen without combustion, the process releases volatile components and creates new substances.
Most of the aromatic precursors are derived from non-volatile compounds, like sugars, amino acids, organic acids and phenolic compounds, which undergo thermal fragmentation without oxygen.
Consequently, the process generates carbon monoxide, hydrogen, ammonia and methanol. Likewise, gas, liquid and sulfurous solids, acetic acid, light oils, tar and methanol.
SEE ALSO: Why does coffee roast color matter?
Summary
| Chemical Changes during the roast (%) | Before Roast | After Roast |
| Caffeine | 1.2 | 1.1 |
| Trigolenine | 1.0 | 1 |
| Amino Acids | 0.5 | 0 |
| Proteins | 9.8 | 7.5 |
| Chlorogenic Acid | 6.5 | 2.5 |
| Sucrose | 8.0 | 0 |
| Reducing Sugars | 0.1 | 0.3 |
| Polysaccharides | 49.8 | 38 |
| Lipids | 16.2 | 17 |
| Caramelization & condensation | 0.0 | 25.4 |
| Volatile Aromatics | 0.0 | 0.1 |
| Water | 8-14 | 1-5 |
| Other Changes | ||
| pH | 4.9 | 5.4 |
| Bean Color | Green | Dark Brown |
| Bean Surface | Wrinkle | Stretch and Porous |
| Brittleness | Elastic | Brittle |
| Moisture | Free and bound water | 13-20% water loss |
| Aroma | Not present | present |
| Density | 600-830g/l | 300-460g/l |
| Organic Losses | chlorogenic and acetic acids, carbohydrates, trigolenine, amino acids |
follow me at:
